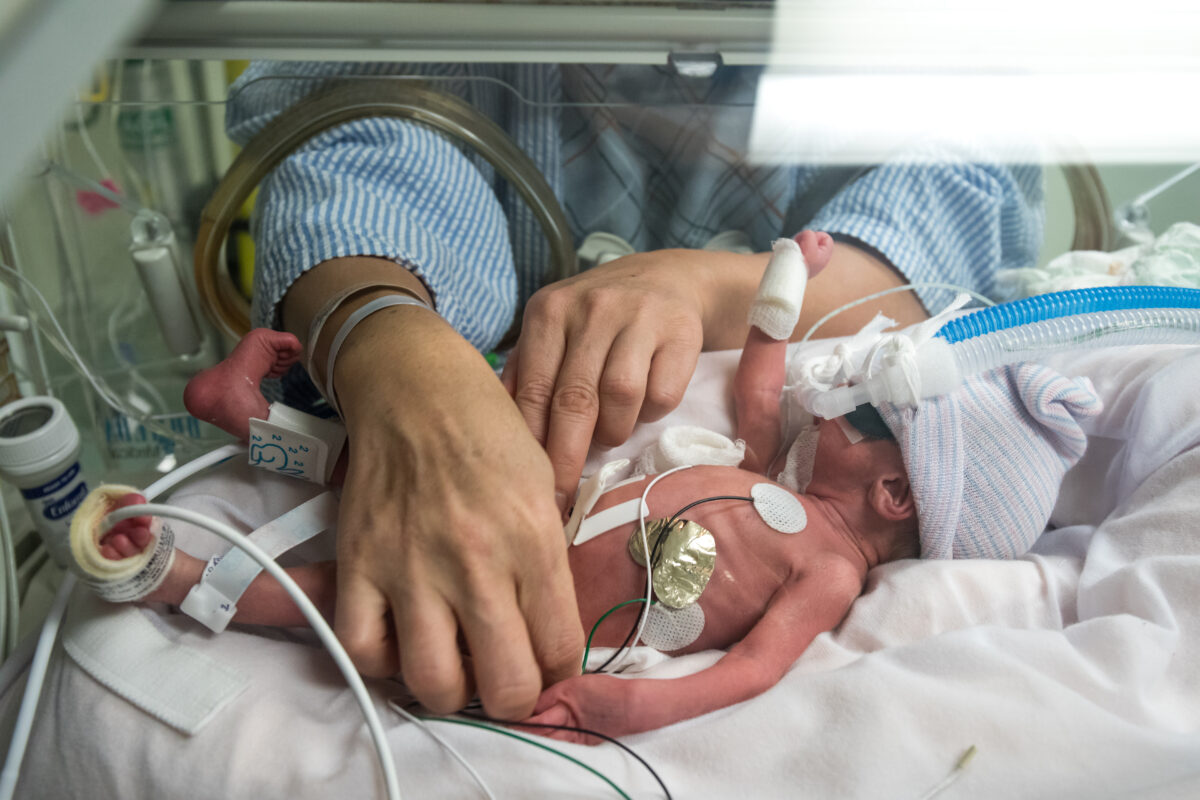
Global neonatal key opinion leaders conclude that prediction of CLD at birth may prevent or slow disease progression – emphasising the need for a rapid point-of-care test.
LONDON, May 25, 2022 /PRNewswire/ — A team of top clinicians specialising in neonatal respiratory disease, and led by SIME Diagnostics co-founder Professor Henrik Verder, have completed a comprehensive review of diagnostic and treatment options for Chronic Lung Disease (CLD). Also known as Bronchopulmonary Dysplasia (BPD), CLD is a major cause of morbidity and mortality in premature babies. The ground breaking peer reviewed paper, entitled “Bronchopulmonary dysplasia with focus on early prediction and treatment: a narrative review“, has been published in Pediatric Medicine [https://dx.doi.org/10.21037/pm-21-98].
PREDICTING CHRONIC LUNG DISEASE WITHIN THE FIRST DAY OF LIFE
The authors evaluated all available and experimental therapeutic approaches to CLD and concluded that predicting CLD within the first day of life is a critical requirement for optimal treatment success. However, current diagnostic methods only confirm a CLD diagnosis after 28 days of life and therefore, treatment is often late and/or sub-optimal. The only known rapid method for predicting CLD at birth is a cutting-edge test developed by SIME, a Clinical AI company specialising in data driven and rapid ICU diagnostics. The company’s breakthrough method for predicting CLD at birth was recently validated in a multi-centre clinical study.1 The test, which combines biometric data with spectroscopic AI analysis of a gastric sample (routinely extracted at birth), has been clinically proven to predict CLD in under 15 minutes.1 This novel point-of-care method was awarded the Chiesi Poster 1st Prize Award Medal at the prestigious Recent Advances in Neonatal Medicine 2021 conference.2
EARLY AND TARGETED TREATMENT CAN REDUCE THE SEVERITY OF CLD
The reviewers concluded that predicting the likelihood of CLD is a prerequisite for the optimal success of established targeted treatments such as: steroids, antibiotics, caffeine, surfactant, nasal continuous positive airway pressure (NCPAP), oxygen saturation targeting and nutrition. In addition to guiding treatment, a predictive CLD diagnostic will also reduce the time and cost associated with drug development. Helping to accelerate the development of novel treatments such as: stem-cell therapy, new recombinant surfactants, vitamins and anti-inflammatories. Ensuring that the next generation of therapeutics are available for patients sooner.
Every therapeutic approach, whether new or established, is associated with levels of risks and multiple adverse events. Furthermore, newborns are very fragile and minimising invasive procedures is a priority for every NICU team. Therefore, it is critical that premature babies only receive treatments that are essential. This need for minimal intervention must be balanced with the significant short and long-term benefits of early and preventative treatment. Predicting CLD at birth will enable neonatologists to create targeted and personalised treatment plans at birth. Reducing unnecessary risks whilst significantly improving clinical outcomes.
FIRST OF IT’S KIND NEONATAL RESPIRATORY CARE PLATFORM
A CLD predictive test will be the second application on SIME’s intensive respiratory care platform. The first application, a rapid test for neonatal Respiratory Distress Syndrome (RDS)3 will be deployed to a select group of world leading NICUs at the end of 2022 as part of an initial product launch.
In the future, NICU clinicians will have a combined rapid screening tool for the two most severe and life-threatening respiratory diseases in neonatology. Predictive diagnosis of both CLD and RDS at birth, has the potential to change the standard of respiratory care for millions of babies worldwide.
view full press releaseReferences:
- Henrik, V et al. Bronchopulmonary dysplasia predicted at birth by artificial intelligence. Acta Paediatr; Volume 110, Issue 2 p. 503-509; https://doi.org/10.1111/apa.15438.
- https://www.recent-advances.com/poster-prize/
- Heiring, C et al. Predicting respiratory distress syndrome at birth using a fast test based on spectroscopy of gastric aspirates: 2. Clinical part. Acta Paediatr. 2020;109: 285-290. https://doi.org/10.1111/apa.14831.