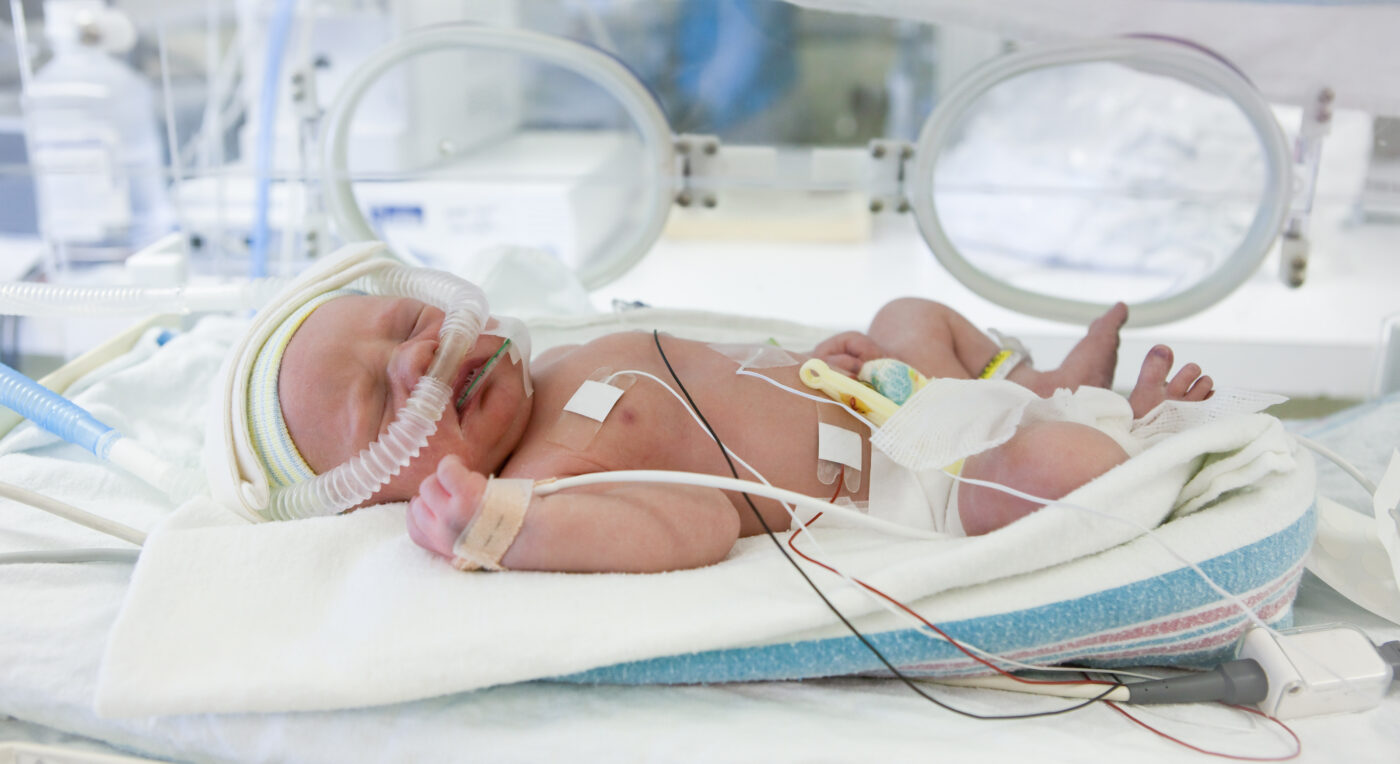
The point-of-care device, which predicts severe lung disease in the ICU and NICU,
has been CE marked for investigational use.
SIME Diagnostics, a pioneer in point-of-care respiratory diagnostics in the ICU, today announced the successful CE-IVD accreditation of the world’s first Clinical AI Platform for the rapid prediction of severe respiratory disease. The CE marked device is designed for use by clinicians, and measures routinely collected samples in <15 minutes via a reagent-free disposable. The thousands of unique datapoints generated by the device are analysed by the platform’s proprietary algorithms to deliver instantaneous results in life critical situations when every second counts.
The company is now starting a pilot of ‘RDS Predict’, the first of several applications that will soon be available on the platform. RDS Predict identifies which new-borns are likely to develop Respiratory Distress Syndrome (RDS), the leading cause of morbidity and mortality in premature babies. By predicting RDS at birth, neonatologists will be able to deliver early and preventative targeted treatment within the first hour of life – improving outcomes and saving lives. This novel screening test is set to change the standard of care for the 15 million premature babies born every year. Pilot sites have been selected from the top NICU’s in Denmark, the UK and the US and a Clinical AI Platform device will be installed at each site for investigational use. The real-world data generated will both support an FDA submission and prepare the company for market entry in 2023.
“The CE Mark is an important milestone for SIME as it demonstrates the efficacy and safety of a much needed tool in acute respiratory care, as well as facilitating the adoption of our Clinical AI Platform in the European market.” said company CEO Povl Verder. “Although both our Platform and RDS Predict have been validated in multiple peer-reviewed clinical trials, this will be the company’s first product pilot in a real-world setting. This pilot, which will be led by neonatal key opinion leaders, will further validate the life-saving potential of RDS Predict whilst generating thousands of new data sets. This data will be used to accelerate the development of novel applications in our product pipeline.”
READ FULL PRESS RELEASE